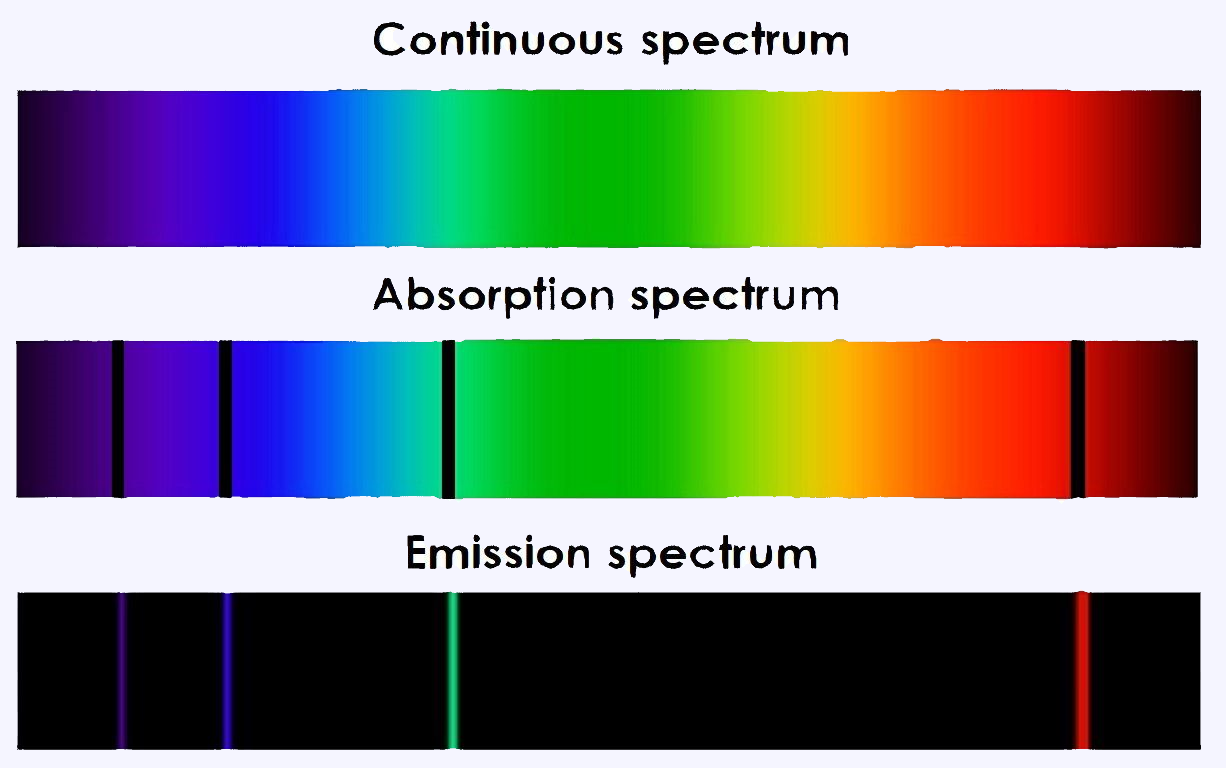
Types of spectrum
Continuous spectrum
When a perfectly white light beam cross through a prism causes dispersion of the light. It gets dispersed into continuous bands of different colours in which all wavelengths and frequencies are represented; this is a continuous spectrum.

Emission spectrum
An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Each of these spectral lines corresponds to a different electron transition from a higher energy state to a lower energy state.
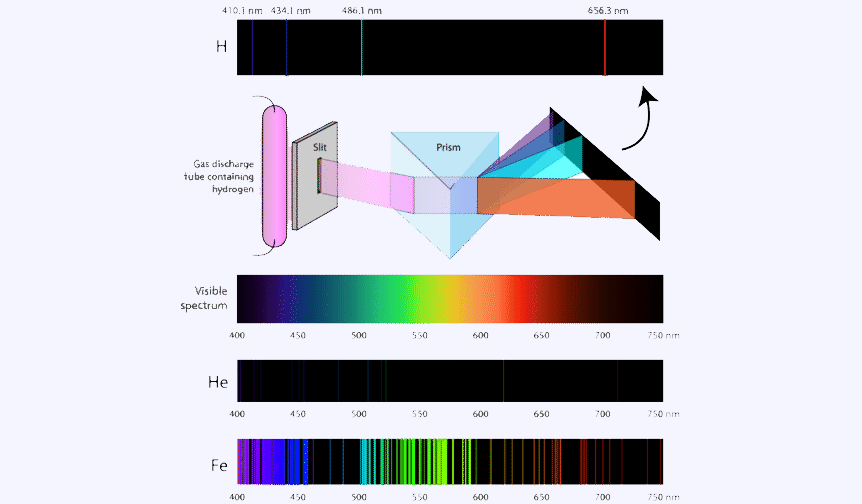
Absorption spectrum
An atom changes from a ground state to an excited by taking energy. This is a process called absorption in which the electron absorbs the energy and goes to a higher energy level. But the atom only absorb specific amounts of energy, so only certain wavelengths of light will be absorbed.
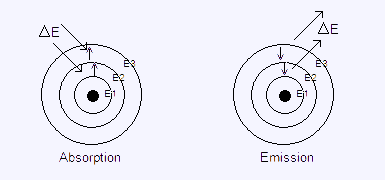
The electron behaviour in the emission and absorption spectrum is:
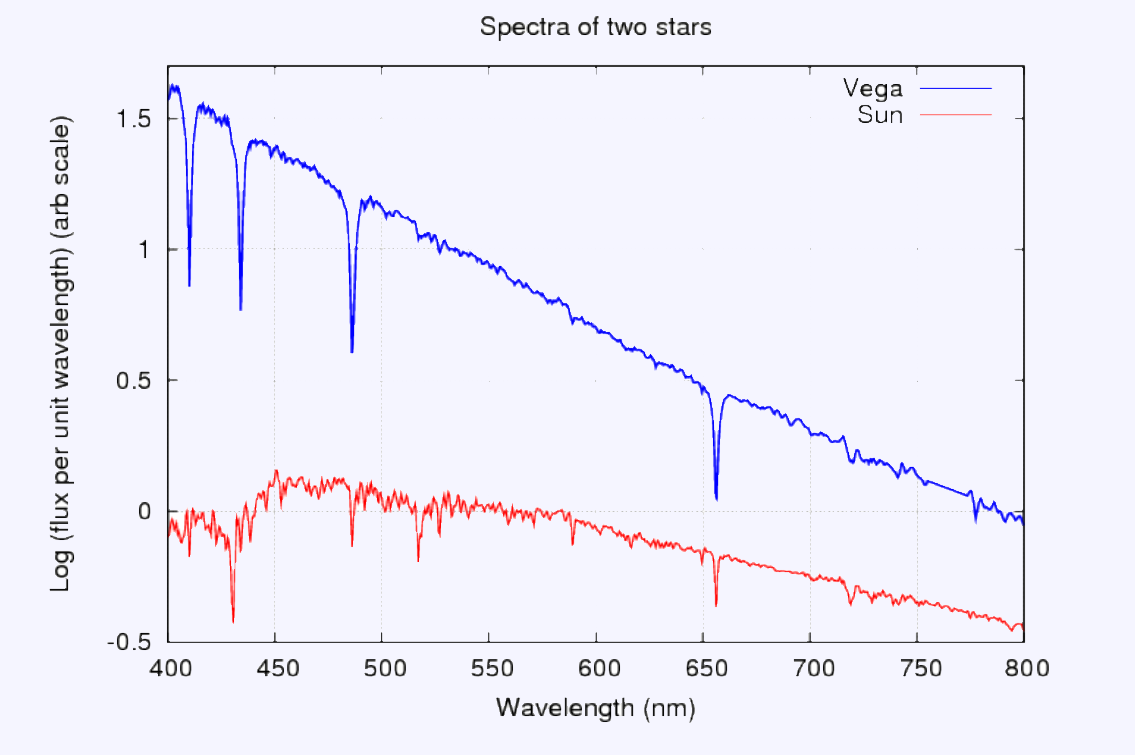
Here are the spectra shown as a graphic. You can clearly see the absorption lines of hydrogen in the spectrum of Vega and Sun. Blackbody is an ideal substance that emits and absorbs all frequencies of light.
Besides that, there is a formula for calculating the energy of absorbed photon. In short, the energy of the photon is Planck's constant times the speed of light divided by the wavelength and it looks like that:
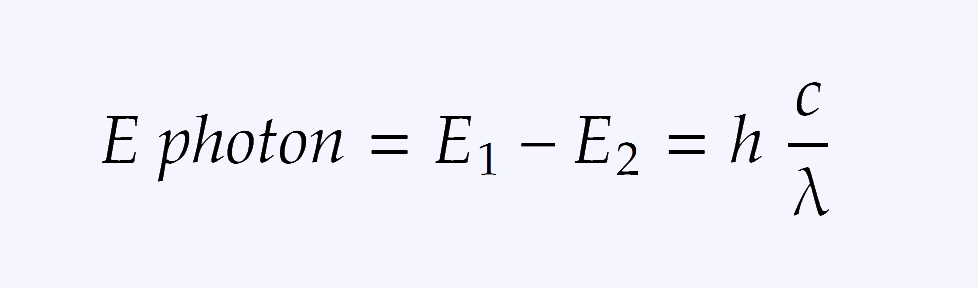
In conclusion, these three types of spectrum can be summarized with the following picture.